How An Ionizer Works
A water ionizer is a small kitchen appliance that separates tap water into two separate streams - one alkaline and one acidic - through a process called electrolysis.
An ionizer is connected to a home’s water supply either through a diverter valve installed on the kitchen faucet or directly through a T-adapter connected to the cold water line under the sink.
STEP 1: FILTRATION :
Water enters the ionizer through an inlet port at the bottom of the unit and is first filtered to remove common pollutants, particulate matter, chlorine, odor, and organic matter present in tap water.
STEP 2: ELECTROLYSIS :
The water then flows through an electrolysis chamber which contains positively and negatively charged platinum-covered titanium electrodes. These electrodes ionize the soluble minerals in the water: positively charged ions gather at the negative electrode to create alkaline water, also referred to as "reduced water" while negatively charged ions gather at the positive electrode to make acid water, also known as "oxidized water".
Alkaline water, which comes out of the top spout on the ionizer, is the fraction that we drink and cook with. It contains a high concentration of positively charged minerals that are beneficial for our health, such as calcium, potassium, and magnesium.
Acidic water, which is dispensed through the bottom hose or spout, is used externally for cleaning and disinfecting the skin and household surfaces. It is discharged into the sink when it is not needed or saved for later use.
How does ionization affect the molecular structure of water?
Water that enters the electrolytic cell is subjected to a small electric current which passes between the plates, causing the water molecules [H2O] to split into two ions: a negatively charged hydroxyl ion [OH-] and a positively charged hydrogen ion [H+].
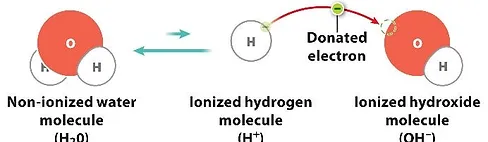
At the exit of the electrolytic cell, the water is separated into two streams ?
The alkaline water stream contains a larger proportion of hydroxyl ions [OH-] which act as a powerful antioxidant, or reducing agent because these ions have spare electrons that can easily be donated to our cells. Antioxidants neutralize the oxidative damage caused by electron scavenging free radicals in our body. The acidic water stream contains a larger proportion of hydrogen ions [H+] which, contrary to hydroxyl ions, act as a powerful oxidant or disinfectant capable of killing bacteria and other pathogens on contact (when pH is below 2.7).